Arkansas Field Crop Pest Management
Arkansas growers of row crops rely on research-based information to help utilize contemporary IPM strategies: transgenic crops, including herbicide-resistant and insect-resistant crops; mitigating development of herbicide resistance in weeds; cultural practices; diagnosis and scouting; and using crop rotation to delay insect and weed resistance to pesticides.
University of Arkansas System Division of Agriculture crop IPM extension faculty members partner with research scientists and county agents to develop and deliver needed information to growers, consultants, and industry representatives. IPM is an essential part of row-crop production, helping producers farm more efficiently and reduce reliance on pesticides.
Evolving methods - bioinsecticides
Pest management tactics are constantly evolving. Among the tools now available to producers are biopesticides, including helicoverpa NPV, a virus that specifically targets only bollworms and budworms that afflict a range of commodities. Read more about its use in managing pests with the fact sheet (pictured): "What You Need to Know... To Successfully Use Viruses for Control of Corn Earworm in Soybean and Sorghum."
Pests and pest pressures differ among the row crops, so the commodities are listed separately. Users of any pesticide should consult the MP-144 Insecticide Recommendation Guide for Arkansas before applying any pesticide, and must ensure following the label – the label is the law, not just a recommendation.
Managing Insects in Row Crops
Below we list several row crops (corn, cotton, rice, and soybean) and the insects that are typically found attacking the crops. Because most pests are only pests of one crop, you will need to look for the crop system, then the insects listed under each crop. After the crops and their pests are presented, there is a short section on beneficial arthropods.
Corn Insect Management
Many insects may attack growing corn, but economic damage may not occur every year. In years of heavy infestations, any one of several of these insects may cause a loss in yield. To prevent loss, you must have knowledge of the insects and the most practical controls. When yield potential is low or other factors are involved (price, etc.), insecticide may be impractical. In such cases, harvesting the crop as silage or fodder may be the best means of salvage.
Several aphids and leafhoppers transmit plant viruses which cause diseases known as maize dwarf mosaic and maize chlorotic dwarf. Insecticide control of these insects is not an effective method of controlling the diseases because of the rapid transmission, reproduction, and infection by the insects. The most effective means of control is the use of disease-tolerant varieties (disease ratings for current corn hybrids are available from the respective seed companies), early planting, and effective control of johnsongrass which harbors the viruses.
Early Planting to Reduce Insect Damage
Insect infestations are normally higher in late summer so that late planted corn is usually subject to more severe damage than early planted corn. Budworm damage is usually light until July and August. Infestations of first generation southwestern corn borer are much lighter than second and third. Corn earworm damage to ears is lighter in early planted corn. Make every effort to plant corn in Arkansas by the first of May.
Scouting Guide
A Guide for Scouting Insects for Field Corn in the Mid-Southern U.S. - AG1286 (PDF)
Corn Insecticide Recommendations
Soil Insects
Several soil insects attack corn. They feed on the germinating seed, roots or underground stems. The most important of these are seed corn maggot, southern corn rootworm, white grubs, and wireworms.
The Southern corn rootworm damages corn by feeding on the root system. The adult Southern corn rootworm female lays eggs around the base of seedling plants in early spring and the larvae move down to feed on the root system. Larvae feeding causes roots to be stubby and tunneling is obvious where larvae have fed. Severe injury may cause plants to lodge and a goose-necked appearance results as plants try to grow erect.
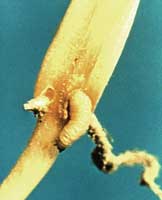
The seed corn maggot is a small white maggot that feeds on corn seed. It can cause stand reduction or loss. The adult flies appear early in the spring and lay eggs on moist soil high in organic matter. The eggs hatch in a few days and the small, white, tapered maggots to feed and burrow into the seed. The feeding may cause the seed to fail to germinate or if germination occurs, the seedlings are weak and may die. Any condition that delays seed germination may increase damage from seed corn maggot since damage is greatest during cool, wet springs. To detect damage, dig in areas where plants have failed to emerge. Once serious damage has occurred, the only alternative is to replant the crop. Systemic in-furrow insecticides will aid in control of seed corn maggots.
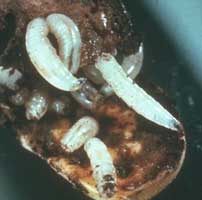
Wireworms damage corn by feeding on germinating seeds and the root system. Wireworm larvae are yellowish brown to brown in color and wire-like in appearance. The larvae mature in 2 to 5 years. Infestations of wireworms tend to be more severe in crop areas following sod. Infestations may be controlled by applying insecticides at planting.

White grubs damage corn by feeding on the root system. The pruned roots cause plants to be stunted and stand reduction may result where heavy infestations occur. Lodging and yield reductions may occur as a result of damaging infestations. White grubs occur more frequently in corn following sod or pastures.

Foliar and Fruit Insects
The corn leaf aphid is occasionally found in large numbers on corn. The corn leaf aphid is a small, bluish-green aphid. The aphids may be found in clusters on leaves and down in the whorl. Control is not normally recommended because infestations rarely cause yield reductions. Aphids are parasitized by a small wasp species and are also susceptible to a fungus disease. Brown, swollen aphids, abnormally larger than other aphids in the colony, indicate parasitism. Several predators also feed on aphids including lady beetles, syrphid fly larvae, insidious flower bugs and green lacewing larvae. These and several other insects prey upon aphids and assist in keeping populations in check. Treatment for corn leaf aphids is rarely necessary.
Several species of cutworms attack corn, but the injury to corn is similar; that is, plants are cut down at the soil line. Cutworms tend to be more of an issue in fields with vegetation, such as cover crops, present prior to planting. Removal of vegetation through tillage or herbicide application should be made 2-3 weeks prior to planting to reduce cutworm numbers. An insecticide application when damage is first noticed will provide control.

Seedling corn may be killed by feeding damage from the corn flea beetle. As the name implies, the beetle hops like a flea. The beetle is small, black, and about 1/16 inch long. It eats holes in the leaves and feeding may severely weaken the seedling.

 - Damage to corn whorl or buds may be caused by the corn earworm and the fall armyworm.
Both insects may be present in the same field. The larvae are similar in appearance,
but the frontal suture (the inverted Y) on the front of the head is more distinct
in fall armyworm. It also has a more greasy and smoother appearance than corn earworm
because of fewer hairs on the body. Corn can withstand a considerable amount of whorl
damage. When infestations are heavy enough to cause dead-heart or severe stunting
of plants, yield loss may occur. When worms infest young plants, two to three feet
in height, they may kill the center of the plant and on seedlings, death of the whole
plant may occur.
- Damage to corn whorl or buds may be caused by the corn earworm and the fall armyworm.
Both insects may be present in the same field. The larvae are similar in appearance,
but the frontal suture (the inverted Y) on the front of the head is more distinct
in fall armyworm. It also has a more greasy and smoother appearance than corn earworm
because of fewer hairs on the body. Corn can withstand a considerable amount of whorl
damage. When infestations are heavy enough to cause dead-heart or severe stunting
of plants, yield loss may occur. When worms infest young plants, two to three feet
in height, they may kill the center of the plant and on seedlings, death of the whole
plant may occur.
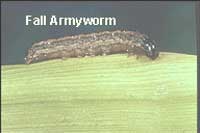 To determine the infestation level, check corn whorls for worms. Control is justified
when you find three to six worms per plant. Several insecticides are effective. It
is important that the insecticide be directed down into the whorl of the corn by using
a high volume of spray material. Thus, ground applications of sprays are usually more
effective than aerially applied sprays.
To determine the infestation level, check corn whorls for worms. Control is justified
when you find three to six worms per plant. Several insecticides are effective. It
is important that the insecticide be directed down into the whorl of the corn by using
a high volume of spray material. Thus, ground applications of sprays are usually more
effective than aerially applied sprays.
The most important insect pests of non-Bt field corn in Arkansas is the corn borer complex, including the southwestern and European corn borers. Yield losses are difficult to assess, but management of these pests has shown potential yield increases of up to 80 bushels per acre. In Arkansas, the southwestern corn borer is the more prevalent of the two species.
Developmental Stages of Southwestern Corn Borer
Egg
- White in color and may be found singly or in small groups
- Up to 300+ eggs can be laid by a single female adult
- 1/16 inch across, elliptical, and flattened on the leaf surface
- Develop 3 orange stripes 36 hours after oviposition
- Hatch in 5-7 days depending upon temperatures

Larva
- Newly hatched < 1/8 inch long with black head capsule and reddish tint on the abdomen
- 1st generation larvae feed on outer leaf layers for about 7-10 days and tunnel into the whorl and stalk
- 2nd and later generations feed on and damage shanks, ears, and stalks
- At maturity, about 1.25 inch long, have a reddish head capsule and numerous black spots

Pupa
- First generation larvae pupate in stalks
- Second generation moths emerge in mid-summer
Adult
- White moths ~3/4 inch long with no patterns on the wings
- Females are slightly larger than males
- At rest, adults fold wings almost parallel to the abdomen
- Adults emerge from corn stubble in spring (May)
- Mating begins within one night from emergence
- Egg laying or oviposition occur two nights from emergence

Damage Caused By Southwestern Corn Borer in Arkansas
- Loss of foliage by first generation larvae is generally minimal and has little direct effect on yield
- Some larvae may tunnel down through the whorl
- If feeding is extensive, the plant may be stunted or killed
- Stalk tunneling is often severe, and as much as 50 percent of the entire length of stalk can be damaged
- Tunneling in stalks may weaken them to the point of breaking and falling across the rows, also known as “lodging”
- Lodged stalks slow harvest operations, and yield can be significantly reduced
- Second and third generation southwestern corn borer larvae also feed on the ear shanks and kernels
- Many fall to the ground before or during harvest, significantly reducing yield
Management of Southwestern Corn Borer in Arkansas
- Plant Bt corn hybrids
- Hybrids containing Bt toxins targeting caterpillars have proven to be very effect at controlling southwester corn borer larvae.
- Early planting
- Plants may reach maturity before second generation larvae impact yields
- Post-harvest stalk destruction
- Can reduce overwintering southwestern corn borer populations by more than 80%
- Foliar insecticides
- Several foliar insecticides available
- Timing is essential for effective management
- Effective timing can be achieved by scouting for eggs and newly hatched larvae
- For a complete list of insecticide recommendations for southwestern corn borer, view MP144 Insecticide Recommendations for Arkansas
- Scouting for southwestern corn borer
- Search for eggs and larvae on leaves.
- Pheromone trapping adults (current pheromone trap catches can be viewed on our Moth Trapping page.
- Helps to effectively time insecticide application
- Growers should consider making an application when traps average:
- 50 moths per trap for first generation borers
- 100 moths per trap for second generation borers
- Several foliar insecticides available

For more information on current insecticide recommendations, please visit MP144 Insecticide Recommendations for Arkansas.
Chinch bugs occasionally attack corn and may cause severe stunting and yield reductions. Chinch bugs are routinely a problem in certain areas of the state and intensive management may be required to control this insect. Adult chinch bugs are 1/6 to 1/5 inch long, and wings on the back are black with white covers crossed with a black zigzag line. Young immature nymphs have red bodies with a white stripe across the back. Older nymphs are darker, lacking the stripe and reddish abdomen. Chinch bugs are normally found near or below the soil line and behind leaf sheaths.
.
Grasshoppers may occasionally be a problem in field border rows. The control of grasshoppers is usually necessary only in localized areas of the field and spot treatments are usually the best way to apply controls.

Sugar cane beetle, also called rough headed corn stalk beetle, is a black beetle about 1/2 inch long. It burrows into the ground and feeds on the corn stem about 1/2 to 1 inch below the soil surface, making a ragged hole in the stem. It is most prevalent in the low wet spots in a field. Infestations do not occur every year. The sugar cane beetle is difficult to control, but insecticides listed for cutworms in help suppress the pest.

Grain Sorghum Insect Management
Insect pests associated with grain sorghum cause a wide range of damage. Foliage
feeding by whorl feeders, such as the corn earworm and fall armyworm, causes a lot
of visible feeding. In reality, whorl feeders cause very little, if any, yield loss.
On the other hand, an infestation of the small sorghum midge causes heavy damage,
but little external evidence shows until harvest. Be aware of the various insect
pests associated with grain sorghum. Efficient management of insects on
grain sorghum requires some knowledge of the biology and seasonal abundance of the
insects. You can get higher yields and save money by managing insect pests based on
biological principles and knowledge of the pest.
Grain Sorghum Insecticide Recommendations
Greenbug Aphid
The greenbug is an aphid that sucks sap from sorghum plants. During feeding, greenbug aphids inject a toxic substance into the plant.
Seedling corn may be killed by feeding damage from the corn flea beetle. As the name implies, the beetle hops like a flea. The beetle is small, black, and about 1/16 inch long. It eats holes in the leaves and feeding may severely weaken the seedling.

 - Damage to sorghum whorl or buds may be caused by the corn earworm and the fall
armyworm. Both insects may be present in the same field. The larvae are similar in
appearance, but the frontal suture (the inverted Y) on the front of the head is more
distinct in fall armyworm. It also has a more greasy and smoother appearance than
corn earworm because of fewer hairs on the body. Sorghum can withstand a considerable
amount of whorl damage. When infestations are heavy enough to cause dead-heart or
severe stunting of plants, yield loss may occur. When worms infest young plants, two
to three feet in height, they may kill the center of the plant and on seedlings, death
of the whole plant may occur.
- Damage to sorghum whorl or buds may be caused by the corn earworm and the fall
armyworm. Both insects may be present in the same field. The larvae are similar in
appearance, but the frontal suture (the inverted Y) on the front of the head is more
distinct in fall armyworm. It also has a more greasy and smoother appearance than
corn earworm because of fewer hairs on the body. Sorghum can withstand a considerable
amount of whorl damage. When infestations are heavy enough to cause dead-heart or
severe stunting of plants, yield loss may occur. When worms infest young plants, two
to three feet in height, they may kill the center of the plant and on seedlings, death
of the whole plant may occur.
 To determine the infestation level, check sorghum whorls for worms. Control is justified
when you find three to six worms per plant. Several insecticides are effective. It
is important that the insecticide be directed down into the whorl of the plant by
using a high volume of spray material. Thus, ground applications of sprays are usually
much more effective than aerially applied sprays.
To determine the infestation level, check sorghum whorls for worms. Control is justified
when you find three to six worms per plant. Several insecticides are effective. It
is important that the insecticide be directed down into the whorl of the plant by
using a high volume of spray material. Thus, ground applications of sprays are usually
much more effective than aerially applied sprays.
Later in the season corn earworm and fall armyworm can also be a problem after grain sorghum heads emerge from the boot. Both species will feed on developing grain and may cause yield loss. Sample for larvae by shaking sorghum heads into a bucket and counting the larvae that fall into the bucket. Treatment is warranted when 1 or more larvae 0.5-inches in size or larger of either species are found per sorghum head.
Chinch bugs occasionally attack corn and may cause severe stunting and yield reductions. Chinch bugs are routinely a problem in certain areas of the state and intensive management may be required to control this insect. Adult chinch bugs are 1/6 to 1/5 inch long, and wings on the back are black with white covers crossed with a black zigzag line. Young immature nymphs have red bodies with a white stripe across the back. Older nymphs are darker, lacking the stripe and reddish abdomen. Chinch bugs are normally found near or below the soil line and behind leaf sheaths.
.
Cotton Insect Management
Effective management of cotton insects requires not only knowledge of their life cycles and biology, but also an understanding of the methods used in determining insect populations in the field. Timely scouting of fields, preferably twice weekly, provides information on the cotton plant and cotton insect populations.
Use treatment guidelines to determine the need for insecticides. Beneficial insect populations are greatly reduced by single applications or eliminated by several applications. Insecticides applied unnecessarily often generate bollworm, aphid, or spider mite problems because of reduced beneficial populations.
Treatment recommendations for certain cotton pests can be found in the MP144 Insecticide Recommendations for Arkansas. Copies are available at your local county extension office. Contact your county agent for assistance in identifying cotton insects and determining the best method of control.
Description
The small white eggs, which hatch into larvae, are deposited inside larger squares. The square drops off the plant as a result of damage from larval feeding. The larva is a small white grub about 1/4 inch in length when mature. The larvae feed for 7 to 11 days and develops completely inside the square into the pupal stage. The pupa is creamy white initially and resembles the adult, but is inactive. As the pupae develop, they begin to show color changes - first black eyes, then just before emergence as an adult they turn brown or tan overall. The adult boll weevil emerges from the pupal stage. The newly emerged adult boll weevil is a reddish - brown and the older overwintered weevil is gray. Both are about 1/4 inch in length and have a beak or snout that is about 1/2 as long as the body length. Two large spines occur on the front pair of legs.
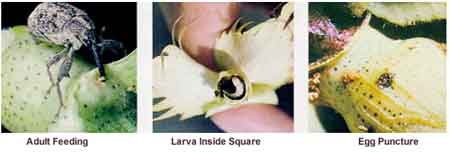
Biology
The adult boll weevil overwinters near cotton fields in wooded areas, along ditch banks, and around old houses or similar areas that have ground debris to protect them. In the spring they return to the nearest cotton field. This results in the first boll weevil damage normally being near overwintered areas. The female weevil lays an average of 150 eggs. In the egg laying process, the female weevil eats a hole in a square or small boll, lays a single egg inside, and then seals it with a gum-like secretion. This secretion dries and appears as a small yellowish bump or raised area on the square. The hatching eggs cause squares to flare and drop to the ground in 5 to 7 days. The average time to complete the life cycle from egg to emergence of adult is 21 days. In midsummer the life cycle may be as short as 16 days.
Damage
The boll weevil adult causes damage to squares with buds 1 /4 inch or larger in diameter and to small bolls by feeding and laying eggs inside them. The damage done by adult feeding or the young developing larvae causes squares to flare and drop. Normally, squares only fed upon will bloom unless feeding is extensive and damages the internal structures - in which case, shedding will occur. Damaged bolls drop or develop abnormally after egg lay or feeding damage. Feeding signs may be recognized by a small hole in the square that turns dark with time. The cotton scout can recognize boll weevil feeding by the yellow to orange fecal material present on the square.
Scouting
Check for damaged squares during whole plant inspection at each sample site. Scouts should pay particular attention to areas next to overwintered sites where boll weevil damage occurs first. Damage continues throughout the season. Count the number of weevil punctured squares found in all samples.
Treatment thresholds are based on the number of punctured squares per row foot. Treatment thresholds are based on the number of punctured squares per row foot.
Description
The egg, larva, pupa, and adult are the four stages of development of the cabbage looper. The small white egg is laid by the adult on the upper and lower surface of leaves. The eggs are greenish - white. They are flattened on the side attached to the plant and this characteristic helps distinguish them from bollworm eggs.
The larva is a light green worm with several white lines down the length of the body. The larvae arches or loops their body as they crawl - thus the name cabbage looper. Three pair of prolegs occur at the rear of the body. The full grown larva is about 1-1 / 2 inches long. They are found mainly on foliage. The pupa is initially a light green turning to a brown color and enclosed in a silken cocoon attached to the plant. The adult cabbage looper is grayish - brown with a wingspread of about 1-1 /2 inches. A silvery white mark resembling a "Figure 8" (that is open at the top) occurs on the middle of the front wing.
Biology
The adult moth lays 250 to 350 eggs that hatch in 2 to 3 days. These small worms (small loopers) feed entirely upon foliage for 14 to 28 days, then pupate. In 12 to 14 days an adult moth emerges from the pupae. A generation is completed in about 35 days. In addition to cotton, cabbage looper larvae feed upon numerous other plants including soybean, cabbage, tomato, several other vegetables, and ornamental plants. Cabbage loopers are controlled by many natural agents but especially by a virus that causes high larval mortality.
Damage
Cabbage looper larvae damage cotton by feeding on the leaves. They eat the leaf area between the leaf veins leaving the leaf with a net-like appearance. Severe defoliation while there are numerous immature bolls in the field, reduces yields significantly. However, feeding damage late in the season may not cause any loss of yield.
Scouting
Damage from cabbage looper can be detected by watching for feeding damage while walking cotton fields. Cabbage loopers are held in control by several natural agents and seldom cause problems. When observed, they should be reported according to the criteria described below.
None - No cabbage loopers present.
Light - An occasional cabbage looper present. Slight defoliation of leaves is observed.
Medium - Several cabbage loopers observed on each plant with 10 to 20% defoliation present.
Heavy - Many cabbage loopers found. Greater than 20% defoliation is observed.
Description
The aphid is a soft bodied insect with all stages ranging in color from light yellow to dark green or almost black. The aphid is a very slow moving insect that secretes honeydew causing the leaves to be sticky or shiny.
Biology
The aphid reproduces very rapidly during favorable conditions. Large populations build up in a short period of time. Females give birth to living young; reproduction is continuous; and there are no distinct generations. A new generation can occur every five days during the summer. The cotton aphid has many natural enemies such as syrphid fly larvae, parasites, and others. Natural enemies and weather conditions help control aphids during the growing season.
Damage
Aphids damage cotton by sucking juices from the plant and by secreting honeydew. High populations in young cotton cause the leaves to curl or crinkle. This causes young plants to become stunted and die. Aphid damage causes leaves to cup downward. When infestation occurs during the main fruiting period, the older leaves may turn yellow and shed. Squares and bolls drop off as a result of severe leaf shed. During late season, honeydew secretion falls on open cotton causing a black sooty mold to develop. In severe cases, the mold stains the lint or causes sticky lint. This is a serious problem for cotton mills.
Scouting
To determine infestation levels, observe aphid populations while walking cotton fields. Classify the population depending on the number present.
None - No aphids present.
Light - Aphids on an occasional plant, 1 to 10 per leaf.
Medium - Aphids on numerous plants and some leaves curling
on edges, 11-25 per leaf.
Heavy - Aphids on numerous plants and leaves are crinkling and curling. More than
26 per leaf are present. A deposit of honeydew is readily visible and the leaves and
plants feel sticky.
Description
 The cotton bollworm and tobacco budworm larvae appear the same externally, but the
adult stage is distinctly different. Some microscopic differences occur in other stages
of development. The developmental stages of both insects include egg, larva, pupa,
and adult. The small white egg is laid by the adult moth and is about the size of
a pin head. Eggs are usually found on plant terminals, stems or top of leaves, but
may be deposited any place on the cotton plant.
The cotton bollworm and tobacco budworm larvae appear the same externally, but the
adult stage is distinctly different. Some microscopic differences occur in other stages
of development. The developmental stages of both insects include egg, larva, pupa,
and adult. The small white egg is laid by the adult moth and is about the size of
a pin head. Eggs are usually found on plant terminals, stems or top of leaves, but
may be deposited any place on the cotton plant.
The larvae range in color from light greenish - yellow to reddish - brown. Several dark stripes occur on top of the body. Length ranges from 1 / 32 to 1-1 / 2 inches depending upon the stage of larval development. Five pair of prolegs occurs at the rear of the body. The pupa is usually found 2 to 6 inches beneath the soil surface. The adult bollworm moth varies in color from reddish - brown to a whitish green-brown. A black or dark spot occurs in the center of the front wing near the forward edge. The hind wing has a dark border along the rear outer edge. The adult tobacco budworm moth has a brown to green color body. The wings have three dark green and off-white bars occurring diagonally across the front wing. The hind wing has a light brown appearance and the wing tips are a darker brown that is edged with white.
Biology
Bollworms and budworms feed on a wide range of host plants including lespedeza, Carolina geranium, tobacco, and cotton. However, tobacco budworms rarely feed on corn, soybean, or grain sorghum which are hosts for the cotton bollworm. The eggs are laid singly and hatch in 3 to 5 days into young larvae. The larvae feed for about 16-17 days on young terminals, squares, and bolls. A generation is completed in 27 to 35 days during the summer months. Many predators and parasites prey upon the bollworm and contribute to its control particularly during the early part of the growing season.
Damage
Bollworms only cause damage in the larval stage. Young larvae usually feed first on terminals and small squares and may sometimes destroy the terminal bud. This results in branching of the plant. The squares and bolls are fed upon extensively by larvae and serious damage occurs in a relatively short period of -time. Larvae feed on 6 to 7 squares and 2 to 3 bolls during their developmental period. Bolls fed upon usually rot and don't develop further.


Scouting
The examination of cotton plants for bollworm and tobacco budworm larvae involves looking for and counting the eggs, larvae, damaged squares, and bolls at each sample site during the whole plant search. Treatment levels are based on average numbers found per row feet. Many of the eggs and young larvae are found in terminal areas of plants around the tender new growth, but the entire plant should be examined for eggs and small larvae. Small larvae feeding on the terminals cause the tender growth to appear brown and slightly ragged. Later in the season, eggs are laid on squares and blooms lower in the plant. Larval damage to squares and bolls is indicated by a feeding hole in the side of the fruiting structure.
Description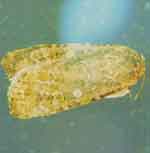
The primary cutworms that attack cotton are the black cutworm, variegated cutworm, and granulate cutworm. The eggs are laid singly or in groups. The larvae vary in color and are usually 1-1 /4 to 1-1 /2 inches long at maturity. The larvae usually feed during the night and hide under clots and leaf trash during the day. The larvae are often greasy-looking and will curl up into a ball when touched.
Biology
Cutworms have one to four generations per year depending upon the species. The eggs are laid singly or in groups on and near the plant. The eggs hatch in 3 to 5 days. The average life cycle is complete in about 30 days, but may range from 2 weeks to 4 months. Many plants are hosts of cutworms including corn, beans, cabbage, peanuts, cotton, tomatoes, and clover. Cool, wet weather is ideal for cutworm damage to occur.
Damage
Cutworms damage cotton in the seedling stage by cutting the plants off at the ground. They also feed on foliage, roots, and stems. Loss of stand to cutworms is the most serious consequence of their damage.
Scouting
Symptoms of cutworm damage are wilted plants or plants cut off at the ground. Cutworm larvae may be found around the base of the plant. Check for larvae in the soil by gently digging soil around plants. Infestations should be reported according to following criteria:
None - No damage
Light - 1 to 5% of the plants damaged with some live cutworms found.
Medium - 5 to 15% of the plants damaged and live cutworms present and cotton stand
threatened.
Heavy - Greater than 15% of the plants damaged, live cutworms present, and cotton
stand significantly reduced.

Description
The fall armyworm has four stages of development - egg, larvae, pupa, and adult. The
eggs are laid on plant foliage in masses of about 50 to several hundred. They are
deposited in layers on the top and bottom of leaves and other plant parts. Egg masses
are covered with grayish fuzz and range in size up to 1/2 the size of a dime.
The young larva is white at first and has a black head. The body becomes dark with
stripes as the larva grows. The larva sometimes resemble bollworms, but have a much
smoother appearance. The larva ranges in color from light green to almost black, but
is usually a light cream colored brown with stripes down the length of the body. The
head is dark and shiny and is marked with a distinctive, light cream colored, inverted
"y" on the front of the head. The mature larva is usually about 1-1 /2 inches long.
The pupa is a reddish - brown color that later becomes almost black.
The fall armyworm adult moth is about 3/4 of an inch long and is dark gray mottled
with light and dark splotches. A noticeable white spot occurs near the extreme tip
of the front wings. The wingspread varies from 1 to 1-2/3 inches. The hind wings are
grayish-white and the front wings are dark gray.
Biology
Fall armyworm attack a wide range of host plants including bent grass, bermudagrass,
cotton, grain sorghum, corn, tobacco, peanuts, and soybean. Fall armyworm egg masses
hatch in two to four days depending on the temperature. Young larvae feed in colonies
for a short time immediately after hatching, but then the larvae disperse to other
parts of the host plant or to other plants in the area. Young larvae often produce
silk-like threads and drop from these threads to other plant parts. Many that drop
to the ground are killed by predators or become infected by soil-inhabiting disease
organisms. Development from egg to mature larva requires two to three weeks. The mature
larva burrows one to two inches into the soil and develops into the pupa. The pupal
stage lasts 10 to 14 days and then the adult emerges.
The fall armyworm cannot survive the winter where the ground freezes. In the spring,
swarms of moths are blown north from the Gulf Coast and the heaviest populations arrive
from midsummer to fall. Many natural enemies attack fall armyworm including a virus
and fungal disease.
Scouting
Check for all armyworm egg masses while walking the field. Egg masses are monitored by examining the upper and lower surface of leaves. Fall armyworm egg masses appear similar to beet armyworm egg masses. Larvae are most difficult to find but frequently are first found feeding on the bolls. Examine bolls for armyworm infestation while conducting the plant inspection for other insect pests. Classify fall armyworm eggs and larval populations according to the classification listed below.
Eggs
None - No egg masses found.
Light - 1 to 4 egg masses found per 56 row feet.
Medium - 5 to 20 egg masses found per 56 row feet.
Heavy - Greater than 20 egg masses found per 56 row feet.
Larvae
None - No larvae found.
Light - 1 to 10 larvae found per 56 row feet.
Medium - 10 - 24 larvae found per 56 row feet.
Heavy - More than 24 larvae found per 56 row feet.
Description
The cotton fleahopper egg is glistening white in color and found embedded in stem tissue. The egg shape is slightly curved, enlarged at one end and has a truncate flat cap at the other end. The small nymph (or immature) is white and translucent at first then becomes a pale green after feeding. Nymphs range in size from very small up to about 1/8 of an inch long. The nymphs have prominent scarlet colored eyes and resemble adults but have no wings. The adult is about 1/7 of an inch long. The pale green body is dotted with tiny dark spots.
Biology
The cotton fleahopper hibernates (or overwinters) in the egg stage. Eggs of the cotton fleahopper hatch in 6 to 12 days and complete development to adult requires 16 to 29 days. Croton is the preferred food plant of this insect. However, it feeds on a large number of other host plants including cotton, horsemint, wild sunflower, and horsenettle.
Damage
Tiny, active, hard-to-see nymphs and adults feed on juices of tender plant parts -especially the terminal buds and small squares. Deformed or ragged leaves are often seen as a result of this feeding. The greatest damage is to small squares that are no larger than a pinhead. They turn brown or black and shed after being fed upon. Heavily infested plants grow tall and whip-like, have restricted growth of fruiting branches, and usually produce only a few bolls near the top.
Scouting
The shake sheet and small square set are the methods used in scouting for plant bugs. The diagnostic key in the color picture section will aid in determining the cause of small square shed. Fields should be scouted for plant bugs until about 2 weeks past peak squaring and when the majority of bolls are set.
Description
The clouded plant bug adult is a tan color and slightly larger than 1/4 inch in length. The first segment of the antennae next to the body is quite large and the first segment of the hind legs is strikingly larger than the front legs. The antennae has prominent black and white alternating bands.
A very young nymph of the clouded plant bug resembles a tiny spider with a small hairy body and long legs. The top of the body is covered with small black hairs and there are red bands on the legs and antennae. The eyes are large and bright red. Larger nymphs are pear shaped, green in color, and have prominent alternating black and white bands on the antennae and hind legs.
Biology
The clouded plant bug has three stages of growth - egg, nymph, and adult. Clouded plant bugs feed on evening primrose, goldenrod, honeysuckle, johnsongrass, morningglory, smart weed, stink weed, cotton, soybean, alfalfa, button bush, black willow, and others. Adult females lay several eggs that hatch in 12-14 days. Eggs are deposited singly by the female in slits made in the plant tissue. The nymph requires about 12-15 days to reach the adult stage. The clouded plant bug overwinters in the egg stage. Three to four generations occur per year.
Damage
Clouded plant bug adults and nymphs feed by inserting their mouth parts into small squares, flowers, bolls, and tender plant tissue. The feeding causes discolored anthers in squares and blooms. Small bolls shed as a result of feeding. Small spots occur on bolls marking the feeding points. The spots are dull black with a pinhead sized, glossy black spot near the center of the larger dull spot. The lint inside the boll may be stained a tan color around the entry point. Malformed bolls may result with damage being done to the entire boll or in tiny spots. Occasional malformation results from improper development of one or more boll locks following heavy anther feeding but no discoloration is usually seen. The symptoms of feeding on vegetative parts are wilted or blackened leaves, lesions, knotted or swollen areas on stems and petioles, malformed or tattered leaves, and excessive branching of the main stem at some nodes.
Scouting
The shake sheet and small square set methods are used in scouting for plant bugs. The small square shed diagnostic key in the color picture section will aid in determining the cause of small square shed. Fields should be scouted for plant bugs until about 2 weeks past peak squaring and when the majority of bolls are set.
Description
The tarnished plant bug has three stages of growth - egg, nymph, and adult. The adult has a brown colored body mottled with small irregular patches of white. On the rear third of the body, along the side, is a clear yellow triangle tipped with a small, triangular spot. The nymph is light green with long antennae and has five black dots on its back. Four of these black dots take the shape of the corners of a square. The nymph has no wings. The nymph moves quickly and resembles the adult as it approaches maturity. The tiny elongated and slightly curved eggs are laid by the adult in small slits made in the cotton stem.
Biology
The tarnished plant bug feeds on many alternate hosts such as alfalfa and other legumes, butterweed, fleabane, goldenrod, aster, and dog fennel. When the weed host becomes unsatisfactory for feeding purposes, plant bugs usually migrate to cotton or other host plants growing nearby. The average time to complete the life cycle is 30 days in summer. Four to seven generations occur per season.
Damage
Tarnished plant bugs feed by inserting their long needle-like mouthparts into tender terminals, squares, and other tissues, and by sucking the juices out. Injured squares usually turn dark and drop off, while damaged bolls may develop abnormally. Terminals fed on may branch out abnormally.
Scouting
The shake sheet and small square set are the methods used in scouting for plant bugs. The diagnostic key in the color picture section will aid in determining the cause of small square shed. Fields should be scouted for plant bugs until about 2 weeks past peak squaring and when the majority of bolls are set.
Description
Several species of mites occur on cotton, but one of the most common is the two-spotted spider mite. Mites are not true insects but are related to spiders. The adult spider mite is very small and about 1/60 to 1/80 of an inch in length. The adult has four pair of legs (insects have three pair). It appears to have two spots on its back, thus the name two-spotted spider mite. Their color ranges from green or orange to straw colored. The adults and nymphs may be seen by looking on the underside of leaves with a hand lens.
Biology
The two-spotted spider mite has a very short life cycle, completed from egg to adult
in five to seven days during the summer. Spider mites pass through the developmental
stages of egg, larva (three pairs of legs), nymph, and adult. Mites feed on cotton
and a wide range of other plants including apple, peach, strawberries, corn, soybean,
various weeds, ornamentals, and others. Two-spotted spider mites have several natural
control agents such as predaceous mites, minute pirate bugs, predaceous thrips, and
diseases that control mite populations. Spider mites feed and reproduce on the underside
of leaves. Initial infestation occurs along leaf midribs.
Damage
Spider mite infestation causes very small yellow spots to appear on the leaves resulting in a speckled appearance. The leaves may turn a red color during dry weather or when the infestation becomes heavier. Areas in fields infested with spider mites may appear lighter in color or reddish from a distance.
Scouting
Spider mite infestation is determined by direct examination of suspected areas while walking fields. Population levels should be classified according to the following criteria.
None - No spider mites present.
Light - Spider mites found on an occasional plant - 1 to 10 per leaf. Some speckling of
the leaves.
Medium - Spider mites are present on numerous plants - 11 to 25 per leaf. The leaves
are speckled and mottled yellow or red.
Heavy - Spider mites are numerous on most plants - greater than 26 per leaf. Leaves are
reddish-brown in color.
Description



The three main species of stink bugs on cotton are the green stink bug, southern green stink bug, and brown stink bug. Stink bugs are shield shaped, about 5/8 of an inch long, and have sucking mouthparts. The southern green stink bug adult is green in color, and the nymphal stage has white spots on the back or abdomen. The green stink bug is also green but the nymphal stage has a striped abdomen. The brown stink bug is brown and closely resembles another predaceous stink bug, the spined soldier bug (a beneficial insect). They can be distinguished one from another by the very sharply pointed "shoulders" on the spined soldier bug. Stink bug eggs are barrel-shaped and are deposited in a cluster on foliage. See the following publication concerning identification of common stink bugs in Arkansas: Identifying Stink Bugs in Arkansas Crops
Biology
Stink bugs overwinter in a variety of habitats such as leaf litter, tree holes, fields,
and other areas. Other host plants include sowthistle, wheat, vetch, peppergrass,
soybean, cowpeas, and wild radish. The egg stage lasts about 4 days; the nymph stage
lasts about 33 days; and the adult stage lasts about 58 days.
Damage
Stink bugs damage cotton by sucking sap from squares and bolls. Affected bolls have
a small sunken black spot on the outside. Internal evidence of feeding may be seen
when bolls are opened by hand and lint/seeds/carpel walls are examined for signs of
feeding injury. The seeds and lint usually turn brown as a result of stink bug feeding.
Often, wart-like callus growths are present on carpel walls, marking the feeding wounds
and plant's response to injury.
Scouting
When stink bug damage or stink bugs are seen while scouting, check infested areas with a beat sheet by shaking plants and reporting the number of stink bugs found per 6-row feet. Medium-sized bolls can also be checked for symptoms of internal feeding injury. See the latest copy of MP144: Insecticide Recommendations for Arkansas for additional sampling and control tactics.
Description
Thrips are very small insects that may be seen if the plant is shaken onto a white cloth.
Biology
Thrips feed on the tender plant foliage of cotton. The eggs are laid on plant tissue or may be inserted into slits. Eggs, nymphs, and adults are found together throughout the summer. Average time to complete life cycle is two weeks. This results in several generations per year.
Damage
Adult and larvae feed on leaves, terminals, and other tender plant parts. Ragged crinkled leaves with a silvery appearance are typical symptoms of thrips damage to young cotton. Leaves usually curl upward and appear burned along edges as a result of feeding in the terminals. Thrips damage is usually on young cotton and severe damage may stunt cotton growth and reduce yields.
Scouting
When scouting, thrips should be counted after shaking or beating 20 plants onto a cloth or into a box. In addition, damage should be observed and classified according to the following criteria:
None - If no thrips or damage.
Light - If newest unfolding leaves show only a slight brownish
tinge along the edges with no silvering on the underside of these
or older leaves, and you see a few thrips.
Medium - If newest leaves show considerable browning along the
edges and some silvering on the underside of most leaves and you
find 1 to 5 thrips per plant.
Heavy - If you readily notice silvering of leaves, deformed
terminal buds, and the plant is generally ragged looking, the
thrips are numerous.
Description
The adult whitefly is white and measures about 1/6 of an inch long. There are three
narrow brown bands across the wings. It has two pairs of wings and flies readily when
disturbed from resting areas on the underside of leaves. The immature whitefly is
flat and scale-like, about 1/30 of an inch long, doesn't fly, and is found on the
underside of leaves.
Biology
Whiteflies pass through four distinct stages of development. The eggs are laid on
the undersurface of leaves and hatch in four to five days. The young larvae can crawl
short distances but once the beak is inserted into the plant they are stationary until
the adult stage. The time for development to adult is about 18 days. Several natural
enemies prey upon whiteflies including lady beetles, big-eyed bugs, and parasitic
wasps. Alternate host for whiteflies include tomatoes, hibiscus, cotton, and many
other plants.
Damage
Cotton is damaged by adults and immature whiteflies who suck the sap from the plant.
Heavy populations may cause some defoliation. A sugary substance called "honeydew"
is excreted by whitefly larvae and accumulates on cotton lint when bolls are opening.
A black sooty mold may develop on the honeydew which stains the cotton lint. Whiteflies are seldom a problem in Arkansas.
Scouting
Whitefly populations are monitored by observing the number of whitefly colonies in the terminal areas of cotton. When whiteflies are noticeable, count the number of terminals that have whitefly clusters. Express as a percentage of the terminals infested. Record according to the criteria listed below.
None - No whitefly present.
Light - 1 to 45% of terminals with whitefly.
Medium - 45 to 65% of terminals with whitefly infestations.
Heavy - 66% or more of terminals with whitefly infestations.
Rice Insect Management
Many insect and spider species are found in rice fields. Only a few insects are considered pests of rice. Insect pests damage rice by feeding on the leaves, stems, roots, and grain. This section contains a brief description, damage appearance, scouting procedures, and control methods for the major and minor pests of rice. Insecticide application should be considered only when insect numbers reach high enough levels that reduce rice yield or quality. Be reminded that many crop production practices influence insect populations. Better crop management can reduce insect pests and insecticide applications.
Treatment recommendations for certain rice insects can be found in the MP144 Insecticide Recommendations for Arkansas. Copies are available at your local county extension office. Contact your county agent for assistance in identifying rice insects and determining the best method of control.
Aphids
Greenbug - Schizaphis graminum (Rondani) and bird cherry-oat aphid, Rhopalosiphum padi (L.) - Occasional Pests - Crossover Pest From Other Crops From Wheat and Weeds to Rice
Aphid infestations normally do not begin in rice, rather aphids may be numerous in wheat or adjacent weeds and move into rice. 1996 was the first year greenbugs were reported in rice. Some were found in the years since, but the number of fields infested and the densities in fields has decreased. Bird cherry-oat aphids were first reported in rice in 1997, and have been reported each year since.
Rice plants with 1 to 2 leaves have been observed to be killed when 2 or 3 greenbugs fed on the plants. Larger plants can be damaged but more greenbugs are required to cause severe damage or death. Aphids and greenbugs should be treated when the rice stand is threatened and substantial numbers of aphids are present.
Armyworm
Pseudaletia unipuncta (Haworth) (also known as the true armyworm)
Occasional Pests - Crossover Pest From Other Crops From Legumes to Rice
The adult is a moth with a wingspan of about 4 to 4.5 cm. The color of the forewings is brown with a single, small white spot about midway across the width and length of the wing. The hindwings are gray or gray-brown. Larvae (caterpillars) can be either green or brown in color but have a distinctive pattern of longitudinal stripes - a dark stripe along each side and a broad stipe along the back (dorsal stripe). The dorsal stripe has a fine lighter colored broken line down the center. The head is pale brown with green and brown mottling.
Armyworm occurs throughout the US east of the Rocky Mountains and in AZ, NM, and CA.
Larvae often become numerous in wheat just before or shortly after heading. Often nearly full grown larvae leave wheat fields, especially those field that are becoming senescent, and move into adjacent rice fields. Damage usually occurs near the border of the two crops and seldom is a complete rice field infested. Pupation can occur in the rice field. Adults are not known to deposit eggs directly onto rice.
Larvae consume leaves and often eat all above ground parts of the rice plant, especially if the plants are small.
No formal scouting plan is used for armyworm in rice. Growers are encouraged to observe rice fields adjacent to wheat for movement of armyworm between the two crops. Treatment may be necessary if stand reduction is evident.
Grape colaspis (lespedeza worm)
Colaspis brunnae (F.) in AR - in LA C. brunnae and C. louisianae
Occasional Pests - Crossover Pest From Other Crops From Legumes to Rice
Adults are about 5 mm (3/16 inch) long, oval, pale golden brown and the elytra (wing covers) have rows of longitudinal ridges and punctures. Larvae are grubs, stout, white to tan in color and up to 7 mm long with a brown head and cervical shield. Pupae construct earthen cells in the soil. Larvae have 3 pairs of thoracic legs plus fleshy appendages bearing a few apical hairs on the abdominal segments.
Eastern US from Maine to Florida, west to southern Wisconsin, eastern Iowa and Kansas, south through Texas and from Oklahoma westward to southern California.
Legumes such as lespedeza and soybeans are primary hosts of the grape colaspis adults. Multiple generations are common in legumes and the last generation of larvae overwinters deep in the soil. In the spring larvae move upwards in the soil seeking plants. Because soybeans and lespedeza are rotated with rice in the midsouth, grape colaspis larvae often have only rice on which to feed. Some larvae complete development on rice. However, rice is not a host plant that adults use for oviposition.
Larvae feed on roots and the portion of the plant stem between the germinated seed and the soil surface. Larvae eat away parts of the stem (girdle the stem) until only a thread-like portion remains. Damaged plants become yellowed, stunted, and wilted. Under water-stress many damaged plants will die. Plants with two or less above ground leaves are very susceptible to damage by colaspis larvae. With very high densities of larvae rice growers can lose 80 to 1 00% of plants - this level of damage often is when rice follows lespedeza. None to moderate densities are often found in rice following soybeans. Areas of damage are randomly distributed and characterized by 6 to 1 0 inch portions of rows with plants showing damage symptoms.
No formal sampling plan is available for scouting grape colaspis. Often the damage is completed before the above ground symptoms become obvious to growers or is altogether ignored or not seen. If the colaspis is suspected, the soil around plants about 2 to 4 inches deep must be removed and examined. Confirmation of grape colaspis presence or underground damage symptoms is needed to separate seedling diseases and problem soils as the cause of damage.
Grape colaspis has been a problem in rice for many years, especially in silt loam soils that tend to be a little sandy. Recent trends towards conservation tillage in rice are coincident with a higher frequency of problems with grape colaspis.
Long-Horned (Antennae) Grasshoppers
Family Tettigoniidae (katydids) In AR rice field: Conocephalus fasciatus (DeGeer) and Orchelimum vulgare Harris Occasional Pests
Adults of Conocephalus usually are about 1 inch in length and adults of Orchelimum are 1 to 2 inches in length. The body is green with brown wing covers. The antennae are longer than the body.
Southern Canada and east of the Rocky Mountains to Northern Mexico.
Adults and nymphs are most often found among grasses near margins of streams, lakes, ditches, and in rice fields. It is not known if species taken in rice use the plants or soil on levees for oviposition sites. Several generations are possible.
Nymphs more than adults feed on young leaves. This is not believed to cause economic damage. Adults and nymphs will feed on anthers of rice flowers and have been found with 'starchy materials', presumably from rice kernels, in the digestive system. Feeding on anthers and kernels is not believed to cause economic damage.
Long-horned grasshoppers can be sampled with a standard sweep net. The efficiency of a sweep net on long-horned grasshoppers is not well known because adults and nymphs drop or move deeper into the foliage when approached. No treatment thresholds are available.
The food of LHG before it is digested is stored in a portion of the digestive system called the crop. The contents of crops were examined in a season long study. Rice leaf tissue was found at low levels throughout the season, but was found in nymphs more often than in adults. Prior to flowering (anthesis) of rice, LHG had remains of arthropods in the crop. Rice water weevils were a favorite meal of the LHG. Once rice began flowering the frequency of pollen replaced arthropods as a favorite meal.
Does feeding on anthers (pollen containing structures) cause a yield loss in rice? Rice is self-pollinating, that is, the anthers rupture just before or immediately after the hulls open to expose the stigma for pollination. LHG could cause sterile florets, but probably not enough for economic losses. Starchy material was found in LHG very late in the season. Feeding on kernels appear limited and not enough to cause economic losses.
Rice Seed Midges
Order Diptera (flies), Family Chironomidae.
Occasional Pests
Adults midges resemble mosquitoes, but the mouthparts are underdeveloped and adults lack scales on the wing margins and wing veins. Larvae are distinctly segmented and have a nonretractable head with opposable mandibles).
Adults prefer to deposit eggs on open water. Masses of eggs are deposited in strings held together by a sticky mucus-like material that forms a protective gelatinous envelope around the eggs. Eggs hatch in 1 to 2 days. The larvae use silk and bits of mud and debris to build tubes on the soil surface. Larval development is completed in 7 to 10 days. Pupation occurs underwater in the tubes. Adults emerge in 2 to 3 days.
Serious damage to rice is limited to germinating seeds and very young seedlings in water-seeded rice. Larvae feed by chewing on the embryo, root shoot, and emerging plant. Larvae are often found inside the seed.
Water-seeded fields should be checked for midge infestation and damage starting 3 to 5 days after seeding. Checks should be made for midge larvae and tubes, damaged seed and damaged roots and plants. Fields should be checked for midge damage until the plants are about 2 to 2.5 cm (1 inch) in length. The number of viable seed per square foot and whether larvae are present, or the number of plants per square foot are used to determine if a field needs to be reseeded or drained.
Rice Stalk Borer
Chilo plejadellus Zincken (Occasional Pest)
Adult moths have about a 2 to 4 cm (1 to 1 1/2 inch) wingspan. The forewings are white or pale brown with randomly placed black scales. Forewing edges have a row of metallic gold scales and black dots. Hindwings are white or pale brown. Larvae are light brown with one dark brown and one light brown stripes along each side of the body. Mature larvae have a length of about 2.5 to 4 cm.
Commonly found in LA and TX and has been reported from GA on rice and in MN on wild rice (Zizania spp.). The first confirmed infestation in AR was found in Chicot Co. in 1981. Since then the rice stalk borer has been found in all counties with rice in eastern and southwestern AR and including counties in central AR along the Arkansas river to Pope Co. (near Atkins).
Larvae overwinter in rice stubble. Pupation occurs in the spring and adults emerge in May. Eggs are deposited in masses (1 0 to 30 eggs) with the individual eggs overlapping so as to form a pattern similar to fish scales. Egg masses are placed on the leaf blade (top or bottom) and sometimes behind the leaf sheath. Eggs hatch in about 5 days. The small larvae enter the rice plant stem by chewing a hole either behind the leaf sheath or near the base of the panicle. More than one larvae enter the stem from a single hole. The larvae eat the inner stem tissues, grow, and eat into the lower larger part of the stem. Mature larvae chew through tissues until only a single thin layer of tissue covers a circular hole in the stem above the water line. The adult escapes through the hole. Very seldom does more than one larvae mature in a single stem.
Larvae eat inner tissues of the stem and effectively stop any-translocation of nutrients. If infestation is just after permanent flood, the whole plant may die or the central culm may die but not the tillers (deadheart). If the stage infested is just prior to emergence of the panicle (boot stage), the green panicle emerges but soon all the florets turn white (Whitehead). Whiteheads are more numerous on edges of fields, edges adjacent to levees, and in barrow ditches.
Rice Stink Bug
Oebalus pugnax (F.), (Hemiptera: Pentatomidae)
The adult rice stink bug is 8 to 12 mm (5/16 to 1/2 inch) long, rice straw color, pale reddish antennae, slender body, and sharply pointed humeral (shoulder) spines that are directed forward and slightly outward. Eggs are placed in two parallel lines on plants. Egg masses consist of 20 to 40 eggs but have been reported to range from 1 0 to over 60. Eggs are cylindrical with a rounded bottom and are about 1/25 inch long and 1/32 inch in diameter. When first oviposited eggs are green, but as embryonic development begins and ends the eggs become red. Nymphs pass through 5 distinct instars. The first is about 1/25 inch with all parts black except the abdomen which is red with two or three black spots. The 2nd through the 5th show an increase in size and a gradual change to light brown with red markings.
Common in North America east of the Rocky Mountains as far north as southern Minnesota and New York. Found in the northern Gulf Coast of Mexico and possibly in the West Indies.
Adults overwinter in accumulated leaf litter, clumps of grass, or in other sheltered places. In AR emergence from overwintering sites occurs in late April and early to mid May. A series of cultivated and wild host plants are used during the season and are critical to survival and population increases. Cultivated hosts are heading wheat, oats, rye, barley early in the season and grain sorghum (milo) and rice later in the season. Grasses (weeds/wild hosts) are essential to rice stink bug survival. The list of host plants is long but includes barnyardgrass, bearded sprangletop, dallisgrass (Paspalum sp.), vasey grass (Paspalum sp.), lovegrass (Eragrostis sp.), ryegrass (Lolium sp.), crabgrass (Digitaria sp.), broadleaf signalgrass, Panicum spp., Johnson grass, and others. However, females do not place eggs in all cultivated and wild host plants. Longevity (life span) and fecundity (number of eggs) is influenced by the host plants of nymphs and adults. The number of generations each year is estimated to be 4.
Eggs hatch in about 4 days and the nymphs remain clustered around the egg shells. The first instar ingests only water. The 2nd through the 5th instar nymphs feed primarily on seeds. Total time spend as a nymph is between 15 and 28 days. After becoming an adult, females start depositing eggs in about 3 to 4 days. Adult longevity has been observed to average about 50 days. Adults remain active in host plants until a host becomes senescent or cool weather arrives.
Adults and nymphs have piercing-sucking mouthparts. Entry of the stylets (mouthparts for feeding) is facilitated by a salivary secretion which hardens on contact with air and remains attached to the rice grain. It is called a feeding sheath. The feeding sheath is the only external evidence that feeding by rice stink bugs has occurred on a grain. Rice stink bugs can successfully feed on kernels from shortly after fertilization until the kernel is in the soft dough stage. The stage of kernel development when fed upon determines the amount and type of damage. Attack during the early stages stops any further development of the kernel (a yield loss). Attack during kernel fill stages removes a portion or all of the kernel contents (also a yield loss); but pathogens are mediated (vectored) into the kernel by the rice stink bug. Infection by pathogens (bacteria or fungi) as well as enzymes produced by the rice stink bug cause discoloration of the kernel (a quality loss plus breakage loss). The rice industry and grain inspection services group all discolored kernels into a category called "pecky rice". Thus, rice stink bugs cause yield losses and quality losses.
Rice fields should be scouted weekly or twice weekly beginning at 75% panicle emergence and continued for 4 weeks. Avoid scouting from mid-day through late afternoon. Useal5inchdiametersweep net to sample for rice stink bugs. At each sample site, make 1 0 consecutive 1800 sweeps to the front and sides while walking forward and swinging the net from side to side. Hold the net so that the lower half of the net is drawn through the foliage and panicles. Count the number of adults and large nymphs after each 1 0 sweep sample. Repeat samples at several random sites (6 or more). Avoid samples at field margins. Calculate the average number of rice stink bugs per 1 0 sweeps. Apply insecticide if infestation is 5 or more rice stink bugs per 10 sweeps during the first two weeks after heading; or if 1 0 or more per 1 0 sweeps is found during the third and fourth week after heading. If the number of bugs is only slightly below the threshold level or if the field is very large, increase the number of samples to improve confidence in sample estimates. Samples taken during the morning hours of 8 to 1 1 a.m. will improve estimates of rice stink bugs.
Rice Water Weevil
Lissorhoptrus oryzophilus Kuschel (Coleoptera: Curculionidae)
The adult is about 3 mm (1/8 inch) long, brown with olive gray areas and with dark (almost black) areas on the thorax and elytra (wing covers). The beak is short and stout and about as long as the thorax. Eggs are pearly white, 1/32 inch in length, and cylindrical with rounded ends. Eggs are inserted into submerged leaf sheaths. The larvae (sometimes called root maggots) are white, legless, attain a length of about 8 mm (1/4 inch) and have a brown head. Each abdominal segment (2 through 7) has a pair of dorsal hooks on small projections. The dorsal hooks are probably used for locomotion, but are modified spiracles (regulated openings) specifically used to obtain oxygen from plant roots.
Lissorhoptrus oryzophilus is found in the US, Canada, Mexico, and Central and South America. It is not native to CA, but was found there in 1959. Only females are found in CA and reproduction occurs parthenogenetically. Female weevils, presumably from CA, were found in Japan in 1976 and in 1 0 years spread over all of the islands. Korea and northern China (recently) have also become infested with rice water weevils.
Adults enter diapause and overwinter in accumulated leaf litter around trees, in the base of bunch grasses, and in other sheltered places. The indirect flight muscles degenerate while overwintering. Spring temperatures determine the rate of muscle regeneration. Adults fly from overwintering sites and begin feeding on host plants. Adult feeding scars can be found on rice that has just emerged. Oviposition (egg laying) is stimulated by the presence of submerged plants. After a short time in flooded host plants (as short a 5 to 7 days) flight muscles begin to degenerate. Eggs are deposited in the leaf sheath below the water surface. The eggs hatch in 4 to 9 days (dependent on temperature) and the larvae may feed in the leaf sheath for a short time, chew an exit hole, sink to the soil surface, and burrow into the mud. Small larvae can be found inside roots, while larger larvae feed on the roots. Larvae complete development in about 4 weeks. Pupation occurs in an oval, water-tight cocoon attached to a root. The pupal stage lasts 7 to 1 0 days. The time from egg to adult takes from 35 to 40 days in AR, LA, MS, MO, TX and up to 75 days during the cooler periods in CA. In AR most first generation adults feed on rice leaves in July, August, and Sept. and begin to fly to overwintering sites in Sept. A small percentage of first generation adults will deposit eggs in rice, giving a partial second generation. In LA two complete generations and a partial third are present each year. TX, MS, MO, and CA have generations similar to that in AR.
Adults feed on the leaf blade surface causing narrow longitudinal scars that parallel the midvein of the leaf. Leaf scarring can be heavy but rarely even the heaviest scarring will result in economic damage. Larvae feed in and on the roots of rice plants. Feeding reduces root volume and can result in decreased ability of plants to acquire, translocate and utilize available nutrients. Damaged plants most often will not show any symptoms unless root damage (pruning) is severe. Severely damaged plants become yellow and stunted, and will have delayed maturity and reduced yield. Occasionally root pruning will be so severe plants cannot remain anchored in the soil and when disturbed will float on the water surface.
Two methods of scouting are currently available - leaf scar counts and larval counts; in the near future a trap for monitoring adults will be available.
Drill-seeded rice - leaf scar counts. Leaf scars are a result of adults feeding on rice leaves. Begin scouting and using leaf scar counts within 4 to 7 days after flooding. Examine only the youngest leaf for feeding scars on plants at least 6 feed from levee furrows (barrow pit or bar pit) and avoid areas of thin stands. At each stop inspect 40 plants and record the number of plants with a scar on the youngest leaf. Make the decision to stop scouting or continue scouting by using the accompanying Table 1. If a decision cannot be made after a reasonable number of stops, rescout the field in 3 to 5 days. Do not use the leaf scar method on drill-seeded rice for more than 2 weeks after permanent flood. When the percentage of new leaves with feeding scars in a field exceeds approximately 60%, between 85 and 90% of the time the number of larvae per core sample will reach or exceed 1 0 larvae per core (the old treatment threshold).
Water-seeded rice. Begin scouting and using leaf scar counts when plants emerge above the permanent flood. Research on leaf scars in water-seeded rice is progressing and no firm recommendations are available. However, when the percentage of new leaves with feeding scars exceeds 50%, insecticide intervention would be recommended. The reasoning for this statement is not to let rice water weevil infestations get too large on very small rice plants. The leaf scar method can be used in water-seeded rice for up to 2 weeks after permanent flood or longer if an insecticide application was made during the initial 2 weeks after permanent flood.
Larval scouting. Scouting for larvae is the same for drill or water-seeded rice. Plants and soil surrounding roots can be used to determine the infestation level of rice water weevils. The size of the soil sample needs to be 4 inches in diameter and 3 to 4 inches deep if soil is from a silt loam and 2 to 3 inches deep if soil is from a heavy clay. Place the core sample in a bucket with a 40-mesh screened bottom. Wash the soil from the plants by thoroughly stirring the plants in the water. Push the bucket up and down vigorously in the water several times. Some force is needed to dislodge larvae from the roots. Most larvae will float on the water surface. The number and size of larvae can be used to predict how much damage will occur. If small or medium sized larvae are found along with large larvae and the total is 1 0 or above, the field may suffer more damage. If most of the larvae are large (1/4 inch) and root pruning is evident but plants do not appear stressed, the field may not suffer any extra damage. Although no research is presently available to confirm the benefit, application of extra nitrogen (30-40 lb/acre) at mid season may help the plants recover from damage.
Soybean Insect Management
The importance of insect pests in Arkansas soybeans is extremely variable from year
to year due in large part to environmental conditions. For example, hot, dry years
favor many lepidopterous pests such as the soybean podworm and the beet armyworm;
and when drought conditions occur, these pests usually are abundant. Many other lepidopterous
pests, such as the velvetbean caterpillar and the soybean looper, may cause problems
following migrations from southern areas, particularly in concurrence with winds out
of the Gulf region where they are a common problem. Generally, insect pressure is
greater in the southern part of the state compared to northern Arkansas due to warmer
temperatures and closeness to the aforementioned migration sources.
Production practices also have an impact on the occurrence of pest insects in soybeans.
For example, insects such as the Dectes stem borer and grape colaspis usually occur
at damaging levels only in soybean monocultures. Row width can also affect insect
pest pressure. Soybean fields which fail to achieve canopy closure by bloom are the
ones most susceptible to damage by the soybean podworm. Planting date, tillage and
adjacent crops can also have an impact on pest species occurrence. The Early Soybean
Production System (E S P S), which is the planting of indeterminate varieties (M G
III and IV) in April, has gained increasing popularity in the state. Fields planted
to the E S P S are susceptible to pests such as the foliage-feeding bean leaf beetle
and the pod-feeding stink bug complex. Because of the limited acreage in this system,
such fields are a virtual "oasis" as a preferred host for these insects which normally
would be found primarily on wild hosts.
A soybean field in Arkansas will contain millions of insects comprising a multitude
of different species, both pests and beneficials, in a growing season. Proper insect
identification and knowledge of the injury associated with pest species are the keys
to any soybean insect pest management (I P M) program. Secondly, we must be able to
determine the level of pest insects in the field by sampling and assessing the threat
of the pest(s) to the crop using such strategies as percent defoliation, stand loss,
etc.
Finally, it is important to determine what management tactics are available and whether
or not they are economically feasible.
Redbanded Stink Bug
The redbanded stink bug is one of the most damaging stink bugs in soybean. The redbanded stink bug is also one of the most difficult to manage. While this stink bug is a common problem in Louisiana, it has been less common in Arkansas and Mississippi. LSUAgCenter entomologist Jeff Davis shares research on scouting and managing the insect into harvest.
Below is a replay of the ArkLaMiss Emergency Redbanded Stink Bug forum held in Stoneville, Mississippi, on Aug. 17, 2017.
Davis' powerpoint presentation may be downloaded here.
The three types of insect pests found in soybeans in Arkansas are:
1. Foliage feeders, which comprise the biggest group of insect pests,
2. Pod feeders, which are probably the most detrimental to yield, and
3. Stem, root and seedling feeders, which are often the hardest to sample and are
not detected until after they have caused damage.
Some insects, such as the bean leaf beetle, may feed on both foliage and pods but
are primarily considered foliage feeders. The following information on individual
insects is meant to provide the reader with basic information on some of the more
common pests found in soybeans including the injury they cause, important descriptive
information, relevant life history details and management considerations.
Insect scouting is essential in determining pest levels in soybeans. All of the thresholds
used to make insecticide application decisions are based on the number of insects
found and/or the extent of damage caused by insects. Soybean fields should be scouted
weekly. The time period from the onset of bloom (Rl-R2) through physiological cutout
(R7) is especially critical. When sampling, at least four areas in the field should
be chosen at random that will provide adequate coverage of the field. Samples should
be taken from each side of the field to adequately detect early insect infestations
from one side of the field. Samples should be taken no less than 50 to 100 feet from
the edges of the field. Remember, many insects such as grasshoppers, stink bugs and
others often feed on wild hosts before entering a field and can often be detected
on one side of the field which has suitable habitat before dispersing throughout the
field.
In Arkansas two tools are used to sample the field: (1) the drop cloth or shake sheet
and (2) the sweepnet. Each of these methods has advantages and disadvantages.
The drop cloth is also referred to as the shake cloth or shake sheet. The drop cloth
is made of heavy cloth or plastic, 36 inches in length, with a one-half inch or larger
doweling about 42 inches long at both ends. Samples are taken by extending the cloth
with the dowels parallel to the soybean rows and shaking the 3 feet of plants on each
row over the cloth. Insects that fall onto the cloth are then counted and the total
divided by six to obtain the number of insects per row foot. Some keys to
taking good drop cloth counts are:
• Bend the plants gently over the cloth, then shake vigorously to dislodge insects
onto the cloth.
• Minimize disturbance of the plants prior to sampling - that is, do not walk through
the plants to be sampled then turn around and sample the plants.
• Be aware of your shadow. Many insects are triggered to fly by a shadow.
• Check the soil at the base of the plants and the areas just below and above the
shake sheet. Count any insects found.
• Count flying insects first such as stink bugs and threecornered alfalfa hoppers
before they can get away.
• Later in the season, as plants get larger, shake only one row per site. Remember
to divide by three (not six) to obtain the number of larvae per row foot.
The drop cloth is very effective for sampling soybeans. It is easy to use and make.
Sample uniformity is easily maintained. Late in the season, particularly when insect
populations are high, the drop cloth is faster to use than the sweepnet and samples
more of the plant. However, the drop cloth cannot be used effectively on narrow rows
(less
than 19-inch rows), drilled or broadcast soybeans.
Sweepnet sampling is conducted with a heavy-duty 15-inch diameter sweepnet. Swing
the net briskly through the top 15 inches of the canopy. Some of the keys to taking
good samples with the sweepnet are as follows:
• The bottom of the net should be angled up so dislodged insects will fall into the
net. Each
pass of the net through the canopy counts as one sweep.
• Sample only one row per sweep in soybeans planted on 36-inch or greater row widths.
In narrow rows, let the normal arc of the sweep continue through the adjacent row(s).
• Sweeps should be made 2 1/2 to 3 feet apart down the row, and be aware of your shadow.
The sweepnet has several advantages for sampling soybean insects. It can be used on
any row width. It is more efficient to use than the drop cloth in short- to moderate-height
soybeans, once the correct technique is learned. Also, the sweepnet is quicker than
the drop cloth early in the season when insect populations are low. Disadvantages
of the sweepnet are that sample uniformity is hard to maintain, less of the plant
is sampled compared to the drop cloth, it is less efficient than the drop cloth late
in the season, and sweepnets are not readily available.
Integrated Pest Management (IPM) is the use of all available control tactics to effectively keep pests from reaching population levels which will cause economic crop injury. These control tactics may include cultural control, biological control, host plant resistance and chemical control.
Management Tip
Assessing pest population and damage levels is an essential component in developing a sound insect management program. Remember, it is important to check fields at least once per week especially once bloom (R2) begins, and this should be continued through physiological maturity (R 7).
Cultural control of insects involves agricultural practices such as crop rotation,
planting dates, tillage practices, row patterns, etc., which may help in the control
of a pest. It is important to remember that such practices must be in harmony with
agronomic practices that promote maximum economic yield.
Tillage such as disking, chisel plowing or other practices can expose many soil insects
to an unsuitable environment. Insects such as wireworms, grape colaspis larvae, bean
leaf beetle larvae, Dectes stem borer and others may be affected by stirring the soil. The effect of no-till
or minimum tillage on insect pests is not well understood. However, it is theorized
that no-till cultural practices may aggravate problems of soil-dwelling insect pests.
Crop rotation is one method of cultural control that has been proven effective for
control of diseases, nematodes and weeds. However, little is known in regard to insect
pests. Experience in Arkansas seems to indicate that problems with Dectes stem borer and grape colaspis are worse in fields with no crop rotation.
Planting date can also impact the outbreak of an insect pest. However, planting dates
are generally determined by climatic conditions, varietal and economic considerations.
Insect management normally should not play a major role in determining when to plant.
As mentioned earlier, the early planting of MG III and IV varieties often provides
insect pests such as the bean leaf beetle and stink bug with a food source which is
not usually available and may result in pest populations of damaging levels. Late
planting may help in avoiding problems with pests such as the bean leaf beetle and
grape colaspis. However, late planting can extend the growing cycle of soybeans, resulting
in greater potential to pod and seed damage by corn earworm and stink bugs. By avoiding
the extremes of planting too early or too late, it may be possible to avoid some insect
problems.
Row width can have a definite impact on insect problems. For agronomic as well as
pest management concerns, it is critical that canopy closure be achieved by bloom
(R 2). It has long been realized that soybean fields which do not reach canopy closure
by bloom are more susceptible to damage by the corn earworm.
Biological control in soybeans is, for the most part, the conservation and utilization of natural enemies of insect pests to keep them from reaching damaging levels. In the soybean IPM system, the major objective is to allow natural enemies to do their work without disruption from insecticides. Growers who make insecticide applications only when they are absolutely necessary take full economic advantage of the natural enemies. Also, when insecticides are needed, consideration should be given to products that are less disruptive to beneficial insects. Bacillus thuringiensis (Bt) may be used for lepidopteran pests such as velvetbean caterpillar, green cloverworm and the looper complex and can provide good control.
Insecticides should be used as part of an overall IPM strategy. They may be thought
of as a "last resort" to prevent insect damage when cultural and biological controls
have failed to keep insect pests below economically damaging levels, but really should
be integrated with other approaches. When insect pest levels reach economic thresholds
and action
must be taken to avoid economic losses, conventional insecticides have been proven
to provide effective and economical control. The only way to determine if an insecticide
application is necessary is by scouting the field to determine pest levels. Never
assume that if one field is at treatment level; all fields should be treated. Differences
in planting date, growing conditions, stage of maturity and other factors often influence
pest levels. Scout every field.
Correct insect identification is critical to ensure the use of a labeled and effective
insecticide. Always read the label of any pesticide before use. Insecticides are an
important component of any soybean IPM program. The careful and judicious use of the
proper insecticide in accordance with instructions on the pesticide label is crucial
for maintaining an effective insect management program. Treatment recommendations
for certain soybean pests can be found in the MP144 Insecticide Recommendations for Arkansas.
Wheat Insect Management
Wheat in Arkansas is considered a winter crop. As a result, the insects that attack wheat are oriented primarily to the fall and spring time periods. In the fall, the primary insects of concern are aphids, fall armyworms and the initial infestation of the Hessian fly. During the winter months, the Hessian fly will develop during periods of warmer weather and can develop into a serious problem in areas where wheat is produced every year and on the more susceptible varieties. The true armyworm and aphids are of concern in the spring. Armyworms are a problem in the state in late May and early June. Infestations of armyworms can reach high levels in some years and fields should be scouted during this time period. The consequences of the Hessian fly infestation will be evident by the presence of shorter stems, lodging and reduced yields when infestations are significant. Treatment recommendations for wheat insect pests can be found in MP144 Insecticide Recommendations for Arkansas.
In the fall, fall armyworms may be a problem in seedling plants. Fall armyworms feed
on the young plants and eat the plants to the ground, causing a loss of stand. Damage
of this type may occur from emergence until a bad frost or freeze eliminates the threat
of armyworms. Wheat will usually recover from moderate fall armyworm damage. An infestation
level of five to six worms per square foot will justify treatment. Treat only during
the warmer part of the day; most of the recommended materials have limited activity
below 60 degrees F.
In the spring, true armyworms are a threat about the time heading starts to occur.
Wheat is very attractive to the armyworm and thick, vigorously growing fields can
attract high infestations. Occasionally, when wheat starts to mature, armyworms will
move up from leaf feeding and cut the wheat heads from the plant stem. Since this
type of damage can have serious consequences on yield, close field observations are
required. Treatment should be made if head cutting is beginning to occur and armyworms
are present. Scouting of fields for armyworm infestations should occur in the spring
when wheat starts to grow vigorously.
Armyworm Control Recommendations
The armyworm may be controlled using registered insecticides. Refer to the University
of Arkansas Insecticide Recommendations Guide (MP144) for the currently labeled insecticides.
The best time to apply an insecticide would be late afternoon since the armyworm feeds
primarily at night.
Wheat in the mid-south is attacked by four species of aphids. These include the greenbug, bird cherry-oat aphid and the corn leaf aphid. All of these aphid species may occur in wheat fields at any time during the production season. In addition to wheat, all the aphids attack a wide range of grass hosts including all the grain crops. Most aphids that occur on wheat will transmit the Barley Yellow Dwarf Virus (BYDV), however, the bird cherry-oat aphid is the most common vector of this disease in Arkansas . Preventing transmission of BYDV by controlling aphids is difficult to achieve using insecticides. Damaging BYDV outbreaks occur approximately 1 out of 10 years. Therefore, in any given year, controlling the aphids that year will only give you at best a 10 percent chance of preventing BYDV outbreaks with a significant potential to reduce yield. The most effective method of controlling BYDV is avoidance of early-planted wheat allowing infected summer hosts to die before wheat emerges, thus, reducing chances of aphid transmission.
Greenbugs are a serious threat to wheat from planting through early spring. Greenbugs
can damage wheat during this period of time and potentially cause yield reduction.
Feeding on wheat causes distinct damage that is easily recognized by yellowing of
leaves and the occurrence of chlorotic spots. This is the result of the greenbug injecting
a toxin into the plant leaves as it feeds. When infestations are high, plants may
turn brown and die. It is quite typical for greenbugs to be higher in number on sandy
knolls or higher places in the field. The greenbug damage symptoms are usually not
uniform and usually occur in spots across the field. The composite damage from greenbug
populations generally results in yellow areas in fields. Heavily infested plants observed
from a distance may appear similar to plants under drought or water stress. Many of
the yellow-looking areas within typical wheat fields in the Arkansas delta may be
the result of "wet-feet," poor nutrition or other conditions. When yellow spots occur
in wheat fields, the field should be scouted to determine the cause of the problem.
The greenbug is a pale green aphid. When the young aphids become about half grown,
they have a dark line down the middle of the back. This is an internal marking, not
a surface stripe. The dark green marking or line on the back is a reliable identification
characteristic for non-winged forms. In the winged forms the head is brownish-yellow
and there are blackish lobes on the back of the thorax. The medial vein of the forewing
has one branch, whereas it has two branches in the forewing of the apple grain aphid,
English grain aphid and the corn leaf aphid.
During warm weather, beneficial insects, such as lady beetles and parasitic wasps
and flies, have some effect in maintaining greenbug populations in check. However,
the activity of beneficial insects is slowed or may cease when temperatures cool below
60 degrees F, while greenbug activity may continue until temperatures reach 45 degrees
F. For this reason, periodic scouting of wheat fields is necessary in order to determine
the need for control of the greenbug.
Field scouting for greenbug infestations should occur weekly from emergence until
cold weather (temperatures below 45 degrees F) is occurring regularly. As spring temperatures
start to go above 45 degrees F, weekly scouting should resume. It is quite common
for greenbug infestations to first occur on the undersides of the lowest leaves, so
whole plant examination should be used to determine infestations. Table 1 lists the
guidelines on suggested treatment levels for greenbugs.
When treatment is required, treat during warmer periods (above 50 degrees F) to receive
the greatest activity from the insecticide.
Table 1. Treatment levels for greenbugs in wheat.
Number Per Linear Foot Plant Height Time of Season
50 4 - 6 inches Fall and early springs
200 6 - 10 inches Mid-March
300 18 - 20 inches Mid- April
800 30 + inches Mid-May
The bird cherry-oat aphid is probably one of the most common aphids found in Arkansas
wheat. This aphid is relatively easy to identify in the field. The bird cherry-oat
aphid is about 1/16 inch long. The body is usually olive green but may vary from nearly
black to a pale green. A reddish-orange patch occurs at the tail of the insect between
and at the base of the cornicles. This dark spot, reddish in color, is probably the
most distinguishing field character that can be used for identification purposes.
The legs and cornicles are pale green with black tips.
The aphid gives birth to living young and the life cycle is typical of aphids. The
bird cherry-oat aphid does not inject a toxin into the plant and thus does not cause
injury to the plant. Damage seldom occurs from this insect and plants tolerate high
populations without losses. Treatment is seldom required.
The corn leaf aphid is about 1/16 inch long. The body is greenish-blue with darker
spots surrounding the base of the cornicles. The cornicles are short and broad with
a dark spot at the base. The legs and cornicles are black.
Growers are often concerned about this aphid but it seldom requires any treatment.
The corn leaf aphid reproduces rapidly and large numbers are common in some years.
Sometimes they cover entire leaves. Both winged and wingless forms are found, especially
when high populations occur. The insects feed until they are killed by a heavy frost
or until their food plants dry up. Large numbers may be tolerated on small grains
and grain sorghum without loss in yield.
Field tests on the greenbug and bird cherry-oat aphid demonstrate that most insecticides
currently recommended give fair to excellent control. Refer to the University of Arkansas
Insecticide Recommendations Guide (MP144) for the recommended insecticides. The greenbug
was controlled by most insecticides tested but the bird cherry-oat aphid was more
difficult to control. Fortunately, the bird cherry-oat aphid doesn't damage wheat
unless extremely high numbers are present. On the other hand, the greenbug can damage
wheat severely if populations are present but control may be accomplished using most
insecticides.
Insecticide applications should be made when aphids reach treatment levels. Heavy
rainfall and natural parasitism will significantly reduce aphid populations so these
factors should be considered before making insecticide applications.
Grasshoppers may occasionally feed upon the borders of wheat fields. Damage to fields is usually restricted to small areas, so spot spraying may control populations. Refer to the University of Arkansas Insecticide Recommendations Guide (MP144) for the recommended insecticides.
The Hessian fly, Mayetiola destructor, recently has become a major factor limiting wheat production throughout the southern
United States . Wheat is the primary host of the Hessian fly but it also will infest
triticale, barley and rye. Although rye generally is not damaged by this insect. Hessian
fly does not attack oats but can develop on some grasses such as little barley and
wild rye grasses.
Adult Hessian flies are small black flies about the size of a mosquito. Adults live
for about two days, during which time they mate. Females lay about 200 eggs in the
grooves of the upper side of wheat leaves. Eggs are orange-red, 1/32-inch long and
hatch in three to five days. Newly hatched larvae move down the leaf groove beyond
the leaf sheath to the stem where they begin to feed at the base of the leaf. Maggots
become white after the first molt and appear greenish-white when fully grown. After
approximately 14 days in the larval stage, maggots molt into a resting (puparial)
stage. The pupa is often referred to as the "flaxseed" stage because it resembles
seeds of flax. The entire life cycle requires about 35 days at 70 ° F. Newly hatched
larvae are exposed on the leaf surface and are susceptible to disease and adverse
weather conditions, but once larvae move to the stem base they are protected from
natural enemies and the environment.
Maggots suck sap and stunt tillers, possibly by injecting a toxin into the plant.
Feeding by a single larva for several days is sufficient to completely stunt or kill
a vegetative tiller. Stunted vegetative tillers are dark green, do not elongate or
produce new leaves, and usually die after the maggots pupate. Infested jointed stems
are shorter and are weakened at the joint where feeding has occurred. Grain filling
of infested stems is reduced and damaged stems often lodge before harvest.
The Hessian fly is a cool season insect that can function normally at temperatures
as low as 38 ° F. The insect spends the summer months as puparia (flaxseed) in wheat
stubble; therefore, burying stubble can reduce fall populations. The number of generations
during the year is governed largely by temperature. Three to four generations occur
per season in Arkansas . Adults emerge from flaxseed with the first cool rains of
fall, often before wheat has been planted. Consequently, the first generation often
develops entirely on volunteer small grains and weed hosts. A second and sometimes
a third generation occurs in late fall and winter and one to two generations develop
in the spring. The fall and winter generations may stunt and kill seedlings and vegetative
tillers. The spring generation infests jointed stems during or after head emergence.
The most effective method for controlling the Hessian fly is use of a resistant variety.
Unfortunately, few wheat varieties grown in the South are resistant to the biotype
(Biotype L) of Hessian fly that commonly occurs in Arkansas . Crop rotation, destruction
of volunteer wheat and tillage that buries wheat stubble will help reduce Hessian
fly infestations in susceptible varieties.
The best method to reduce injury and damage by the Hessian fly is to delay planting
as late as is reasonably possible. In Hessian fly research, early-planted wheat had
a 21 percent infestation compared to less than 1 percent in the later planting date.
Dissulfoton granules also reduced Hessian fly infestations. Generally, wheat planted
after October 1 in north Arkansas and October 10 in the south, may substantially lower
infestations of Hessian fly. If the Hessian fly is a serious threat, the use of in-furrow
insecticides at planting is effective in protecting the wheat against the first and
perhaps the second field generation of the Hessian fly. Wheat that is planted in mid-
to late September is very susceptible to Hessian fly infestation. The later in the
fall that wheat is planted the safer the crop is from threat of Hessian fly infestation.
A fly-free or low infestation risk planting date probably does not exist in southern
Arkansas where winter temperatures do not limit Hessian fly activity. However, later
plantings in the northern areas of Arkansas have reduced infestations of the Hessian
fly. The risk of the Hessian fly infestation must be weighed against the threat of
wet weather that can prevent the planting of the crop.
The Hessian fly may be controlled in susceptible wheat during the fall by using a
systemic granular or liquid insecticide applied in-furrow at planting. The rates and
insecticides for control of Hessian fly can be found in the University of Arkansas
Insecticide Recommendations Guide (MP144). This treatment, as effective as it is,
will not prevent re-infestation by subsequent generations during the winter and spring.
However, research conducted on in-furrow treatments has shown significant yield increases
when Hessian fly is a significant factor in the production of wheat. Studies have
shown that foliar applications of insecticides in the winter and spring for Hessian
fly control are not effective.
The cereal leaf beetle, which is native to Europe , was first found in the United
States in 1962 in southern Michigan and first found in Arkansas in 1995. The adult
beetle is about 3/16-inch long, metallic blue-black in color with red legs and a red
pronotum (neck). The female is generally larger than the male. Adults begin to emerge
in late March to early April from overwintering habitats, which include crop remnants,
field trash, grain stubble and corn plants. The females mate and then deposit eggs
on the upper surface of wheat leaves. The eggs are long, yellow, cylindrical and turn
darker as they mature. The eggs hatch in 3 to 7 days. The newly hatched larvae are
pale yellow with a brownish-black head and legs. The larvae characteristically cover
their body with fecal material and appear black and slug-like in the field. The larval
stage lasts 3 to 4 weeks and the pupal stage from 7 to 14 days depending on the temperature.
Adults and larvae feed on the leaves of the plant, removing long narrow strips of
tissue between the veins similar to the rice water weevil except the strip is slightly
larger. Fields with heavy damage have a white frosted appearance. The adults and larvae
damage oats, wheat, barley, rye, corn and grasses. Oats are the preferred host with
plant maturity playing an important part in attractiveness and susceptibility.
Control of Cereal Leaf Beetle
Adults and larvae are easily detected in the field by visual examination. The population
of cereal leaf beetles may be determined by randomly selecting 20 stems at five locations
in the field. At each location, examine the flag leaves for the beetle adults and
larvae and count the number of each. The economic threshold for cereal leaf beetle
is one larvae or adult per flag leaf.
Beneficial Arthropods
Beneficial insects and other arthropods (e.g., spiders) and diseases play an important part in the control of crop insects. Their importance often goes unnoticed until they are eliminated by an insecticide application and pest numbers build up as a result.
Beneficial insects in crops consist of two groups: (1) predators, and (2) parasites. Predators catch and eat smaller insects, usually killing them to get a single meal. One predator can destroy many prey. For example, a lady beetle may eat several thousand aphids during its lifetime.
Parasitic insects live on or in the bodies of other insects during at least one stage of their life cycle. The parasite gets food from its host and the host eventually dies. Most of these parasites are either flies or wasps that are parasitic as larvae and free living as adults.
Beneficial insects vary in importance in different areas. Some may be more numerous in one area than another and their numbers vary from year to year. Predators are more conspicuous than parasites and their importance is generally appreciated more. However, both are important in controlling harmful insect populations.
Predators
There are many species of assassin bugs varying from 2/3 of an inch to 1 inch long. They are dark brown in color, but are sometimes also marked with pink or red. Both nymphs and adults feed on the larvae and adults of most injurious insects except thrips, aphids, and spider mites. Some assassin bugs are excellent predators of the large bollworm larvae.
The two species of bigeyed bugs found in Arkansas fields are Geocoris punctipes and Geocoris uliginosus. Bigeyed bugs are black and white, about 1/4 of an inch long, and have large bulging eyes. Both adults and the grayish nymphs feed on bollworm eggs and small larvae, fleahoppers, leafhoppers, aphids, plant bugs, and the eggs and small larvae of various moths.
The two species of Nabis that are common in cotton fields are the damsel bug and pale damsel bug. Nabids are slender, brownish bugs that have slender legs and antennae. They are about 3/8 to 1/2 of an inch long and their long narrow head is armed with a long beak. Nabids feed on fleahoppers, plant bugs, leafhoppers, spider mites, aphids, moth eggs, and small larvae.
Flower bugs are small and inconspicuous and are considered to be one of the most beneficial of all predators in crop fields. Both nymphs and adults of this insect are voracious feeders on aphids, plant bugs, thrips, mites, and moth eggs. The insidious flower bug is an excellent predator of bollworm eggs and first stage larvae.
Lacewings are important predators of eggs and larvae, as well as other soft bodied insects. The larvae are somewhat similar in appearance to lady beetle larvae. The adults are slender green or yellowish - green. Lacewing eggs are placed singly on a slender thread-like stem that is attached to leaves or stems of the plant. The adults of some species are predaceous on moth eggs, but the larvae are the most effective predators. Lacewing larvae can consume as many as 42 bollworm eggs per day.
Ground beetles are common in damp places. Adults are predaceous, feeding primarily at night. They feed on bollworms, cutworms, wireworms, and other lepidopteran larvae. They have been observed climbing the cotton plant to feed on bollworm moths and larvae. Several species of ground beetles occur in crop fields.
Several different lady beetles are common in field crops, including the spotted lady beetle and the convergent lady beetle. Both the larvae and adult stages are beneficial. They attack aphids and spider mites, and destroy the eggs and larvae of the bollworm, cabbage looper, armyworm, and other soft bodied insects.
Many species of spiders are found in field crops, including lynx spiders, wolf spiders, and crab spiders. Since spiders will eat most injurious insects they are extremely beneficial. They catch their prey in webs or spread themselves out on the upper surface of a leaf in the plant terminal and await their prey.
The larvae of syrphid flies prey primarily on aphids. The adults feed only on pollen and nectar. They lay eggs singly on leaves. After hatching, the sluglike, green or tan larvae, consume eggs or young of their prey. One larva may destroy hundreds of aphids before reaching maturity.
Other insects, such an ants, dragonflies, long-horned grasshoppers, predaceous mites, predaceous thrips, brown lacewings, preying mantis, owlflies, ambush bugs, tiger beetles, and soldier beetles, may contribute to controlling injurious insects.
Parasites
These tiny wasps lay their eggs in the bodies of aphids. A few days after they lay an egg in an aphid, the aphid becomes paralyzed. The aphid stops feeding, becomes swollen and discolored, and finally dies. In a few days the parasite emerges from a circular hole in the body of the aphid.
These wasps vary in size and most are brilliantly marked. They can be recognized by their short jerky flights and constantly vibrating antennae. They deposit their eggs and complete their development inside a variety of destructive insects, including bollworms armyworm, and similar pests. In addition to ichneumon wasps, several braconid wasps attack bollworm and budworm larvae.
Parasitic flies resemble an overgrown gray, brown, or black-mottled housefly. These flies attack caterpillars by laying their eggs on them. The eggs hatch into maggots and complete their development inside their host. These parasitic flies attack many Lepidoptera larvae.
Insecticide Recommendations
Insecticide Recommendations for Arkansas
